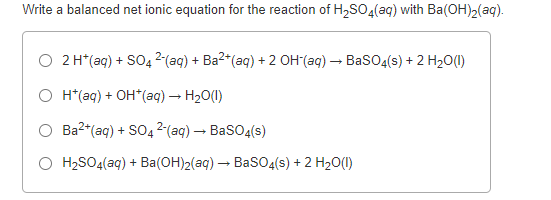Barium hydroxide (Ba(OH)2) and sulfuric acid (H2SO4) react to produce water and barium sulfate (BaSO4) . Write and balance the equation. | Homework.Study.com

OneClass: if 98.0g of H2SO4 is reacted with Ba(OH)2 determine the percentyield of BaSO4 if you isolat...

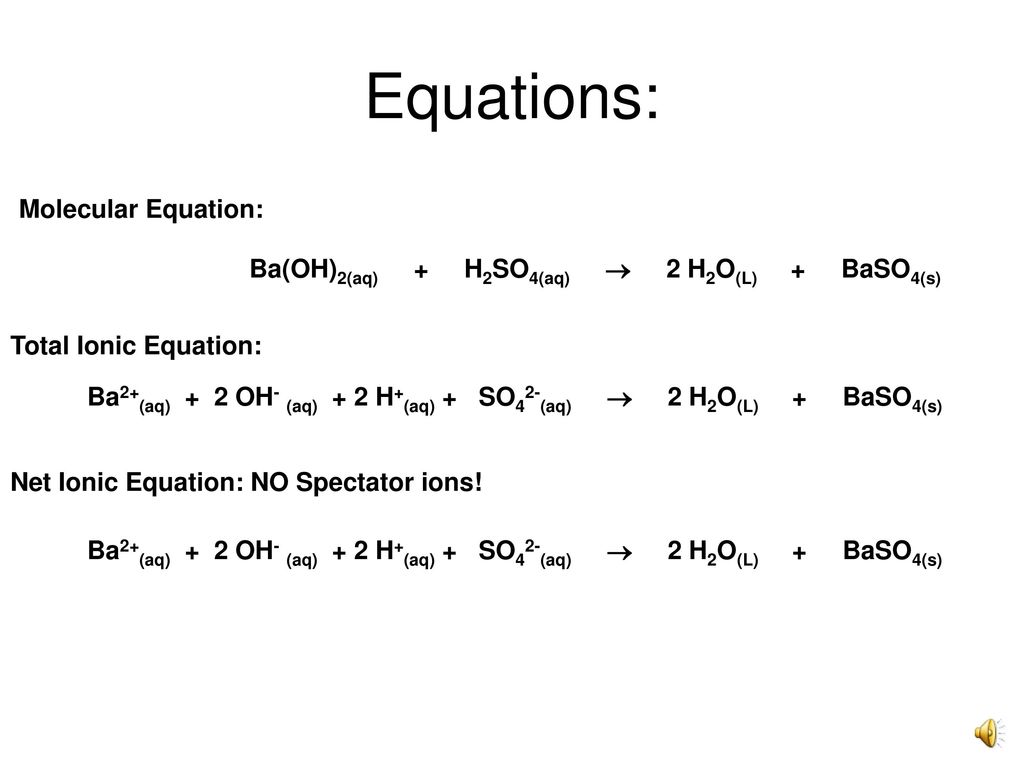
How to Write the Net Ionic Equation for Ba(OH)2 + H2SO4 = BaSO4 + H2O (Note: it should be 2H2O) - YouTube

How to Write the Net Ionic Equation for Ba(OH)2 + H2SO4 = BaSO4 + H2O (Note: it should be 2H2O) - YouTube

Ba(OH)2+H2SO4=BaSO4+H2O Balanced Equation||Barium hydroxide+Sulphuric acid=Barium sulphate+Water - YouTube
25. 100 ml 0.1 M Ba(OH)2 solution is mixed with V ml X M H2SO4. The number of millimoles of BaSO4 ppt is 8 . Find V and X

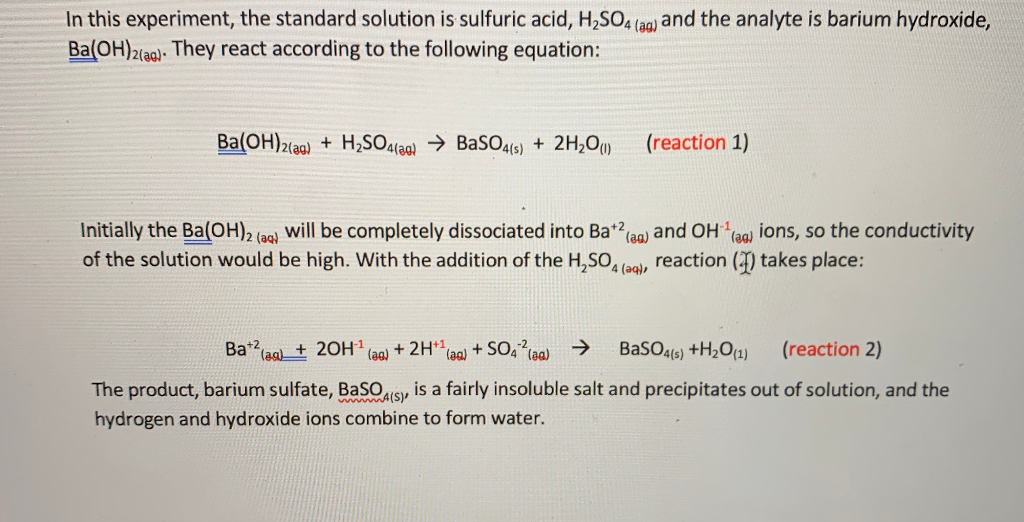
SOLVED: Sulfuric acid (H2SO4) and Ba(OH)2 solutions are strong electrolytes and give a bright glow on the light bulb of your conductivity apparatus. When Ba(OH)2 is added dropwise to H2SO4, a precipitate
22.Assuming that100 cc. of 0.1M solution of H2SO4 is needed to nytralise 200cc Ba(OH)2 solution the normality of Ba(OH)2 solution is?also explain the units 'cc'
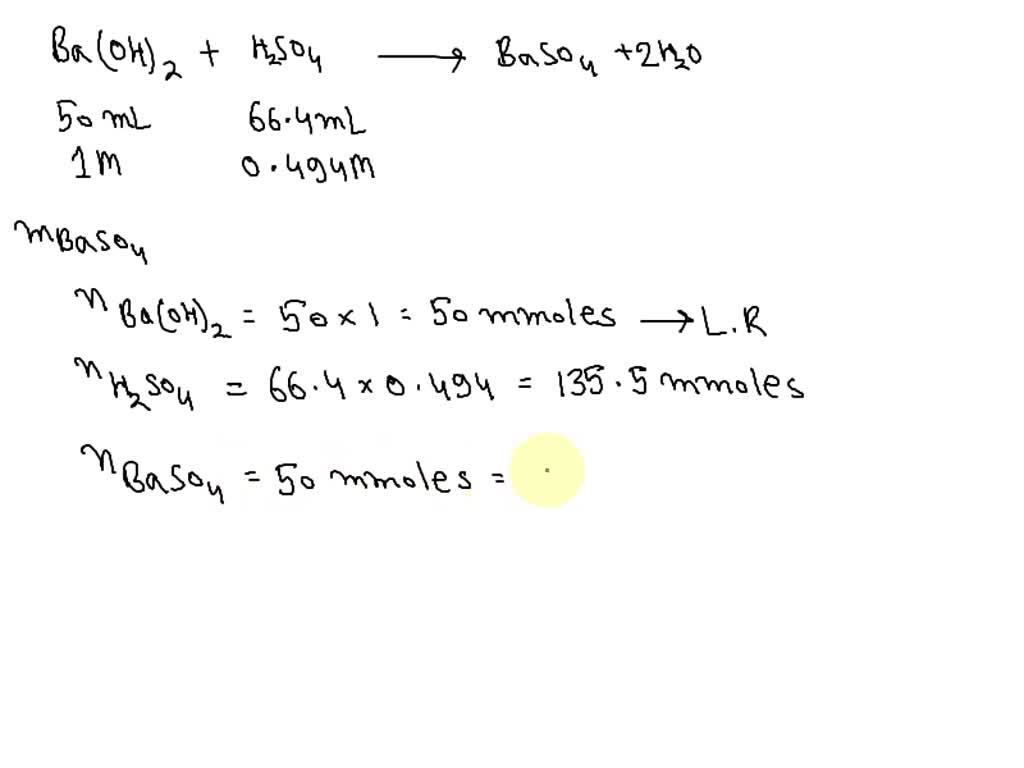
SOLVED: A student mixes 50.0 mL 0f 1.00 M Ba(OH)2 with 66.4 mL of 0.494 M H2SO4. Calculate the mass of BaSO4 formed (in grams). the pH of the mixed solution.