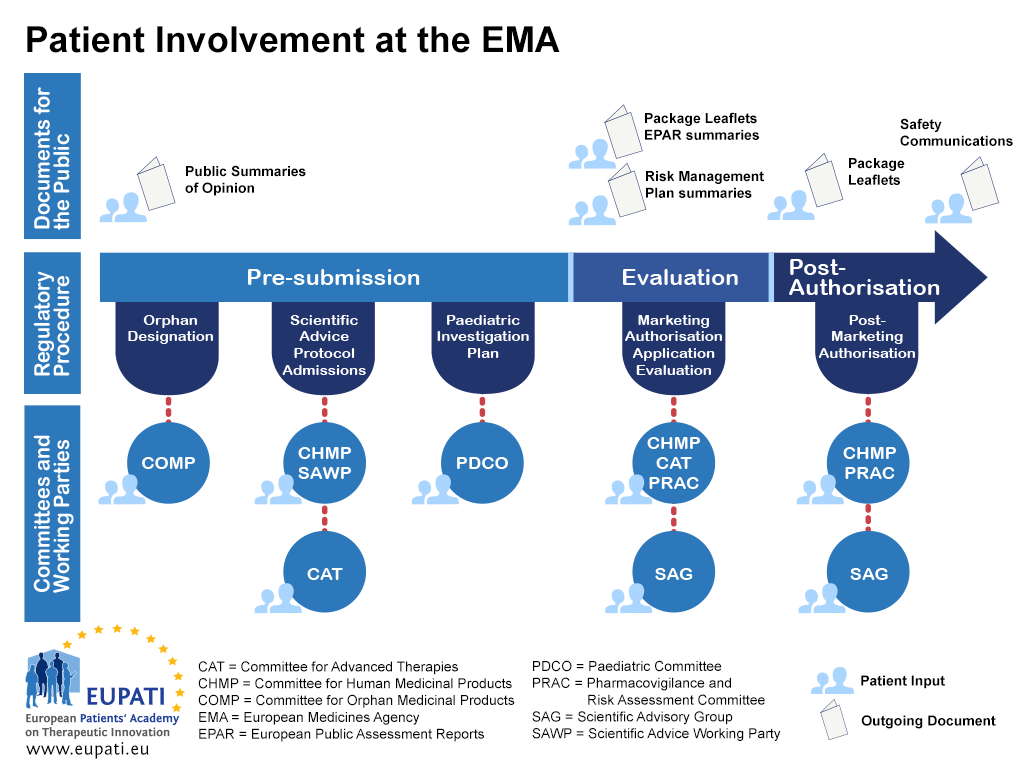
European Medical Agency has released news about consultation on draft guidelines on quality requirements on medical devices in combination products - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety

EU Careers - The European Medicines Agency (EMA) 🇪🇺 has an open job opportunity! EMA is looking for a Lead Assistant to join its team based in Amsterdam 🇳🇱 Find info &

European Medicines Agency (EMA) | CDE Almería - Centro de Documentación Europea - Universidad de Almería

EU Medicines Agency on Twitter: "EMA's safety committee, the #PRAC, starts review of #terlipressin medicines and recommends updates to product information for some #COVID19vaccines: 👉https://t.co/EFGDJII0Xt #SafetyOfVaccines https://t.co/wLcVnqYMDs ...
EU approved biosimilars are interchangeable with reference medicines/ equivalent biosimilar: EMA, HMA, Health News, ET HealthWorld