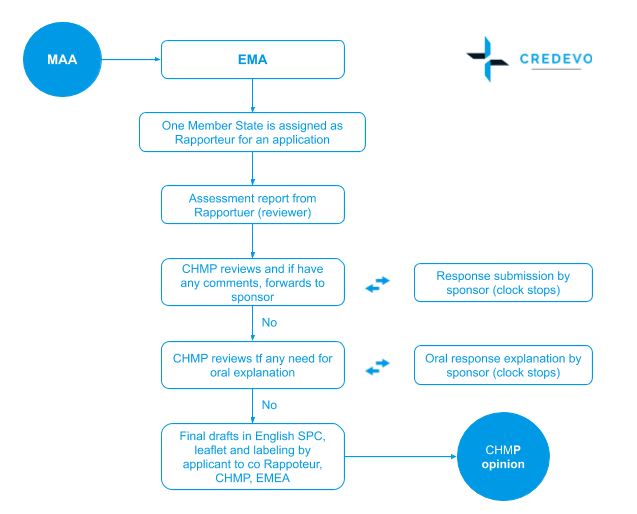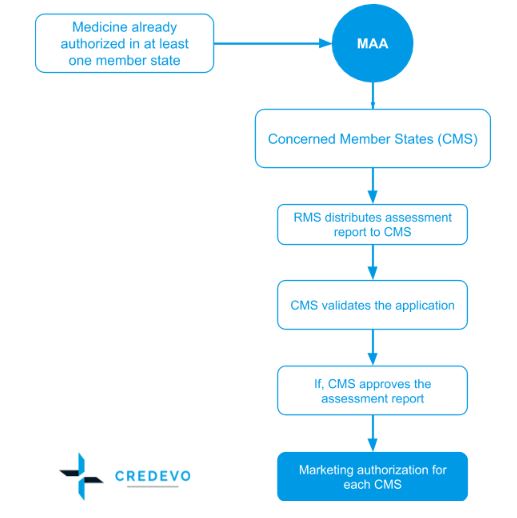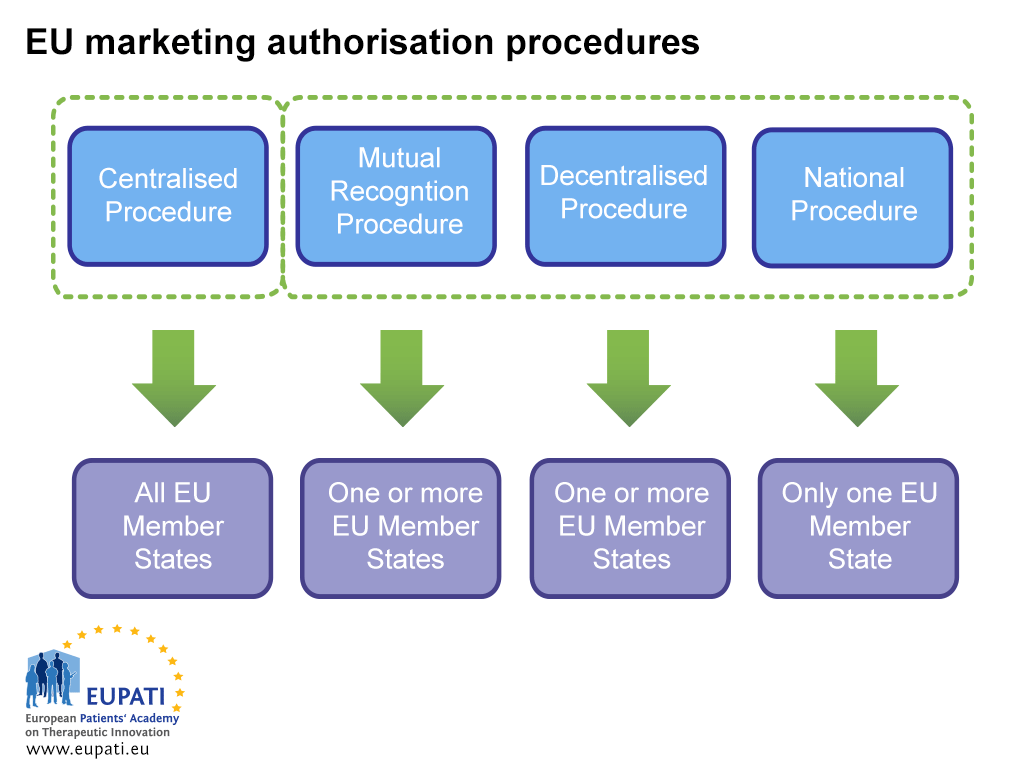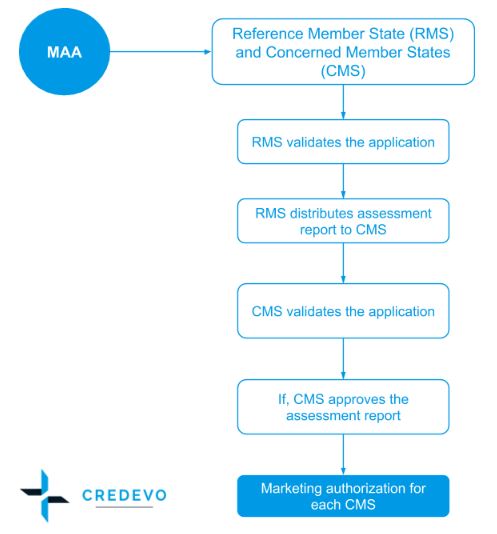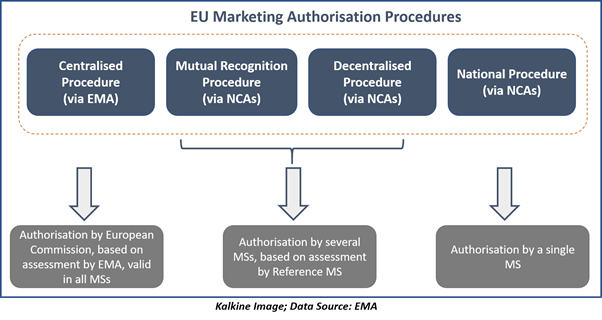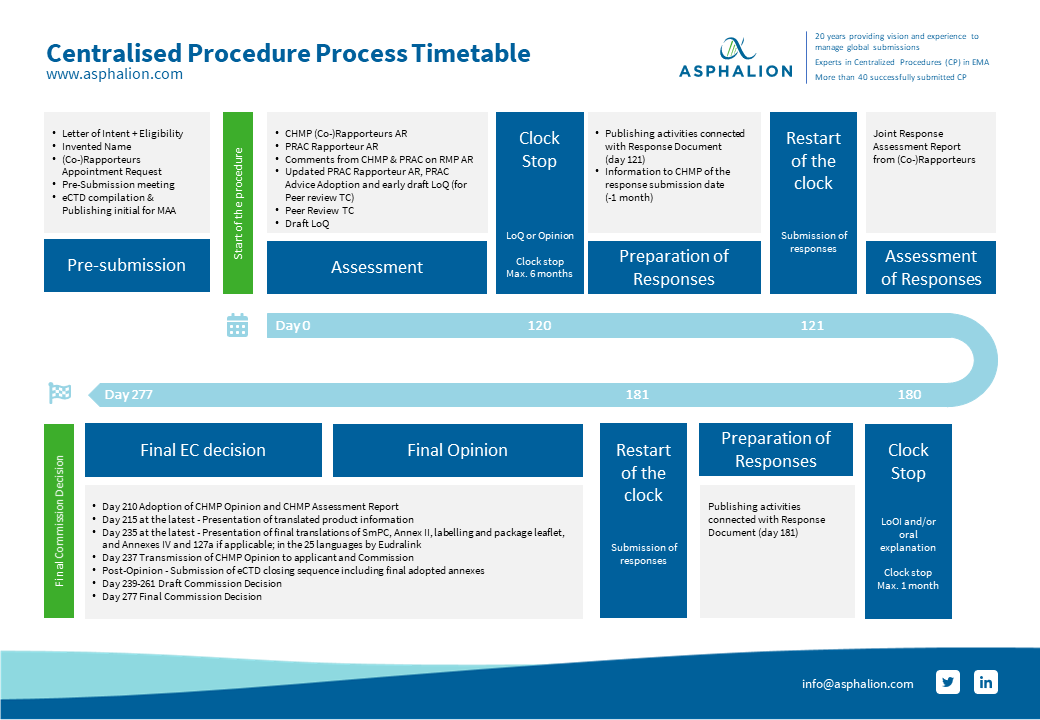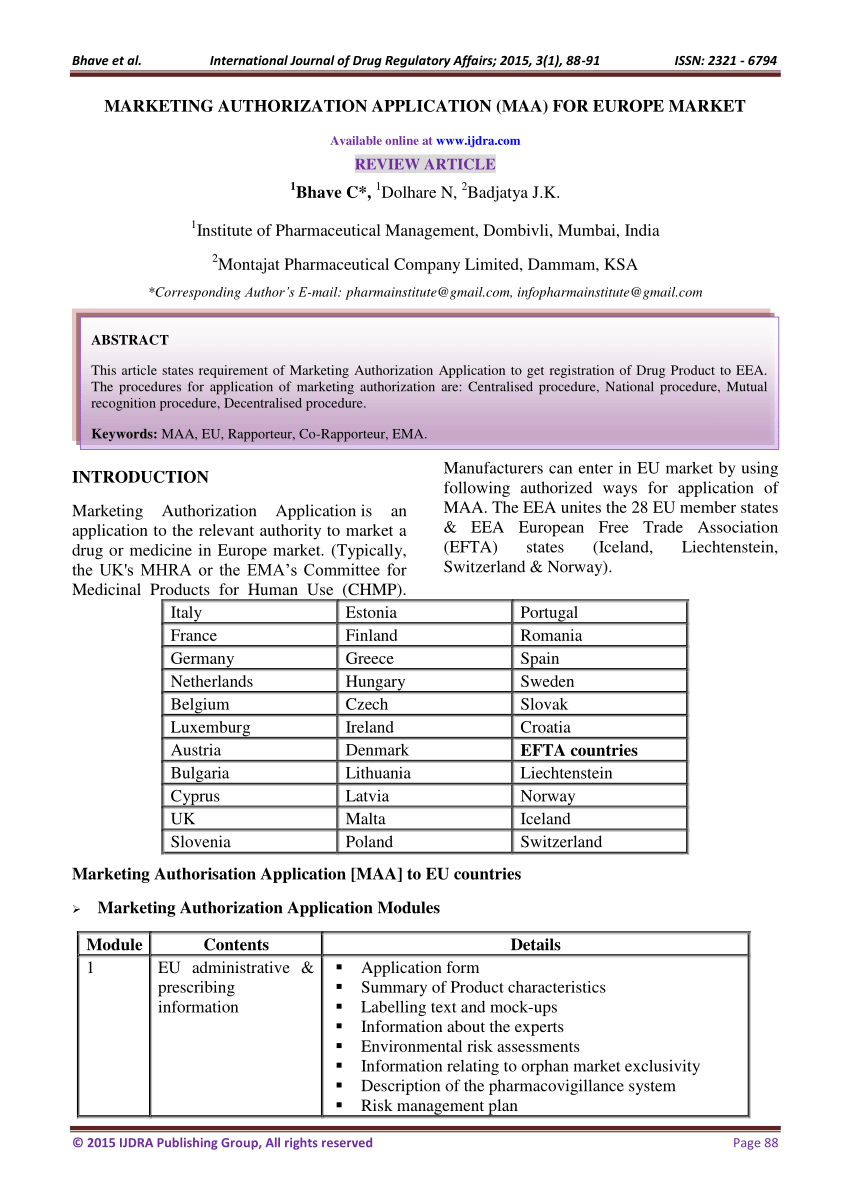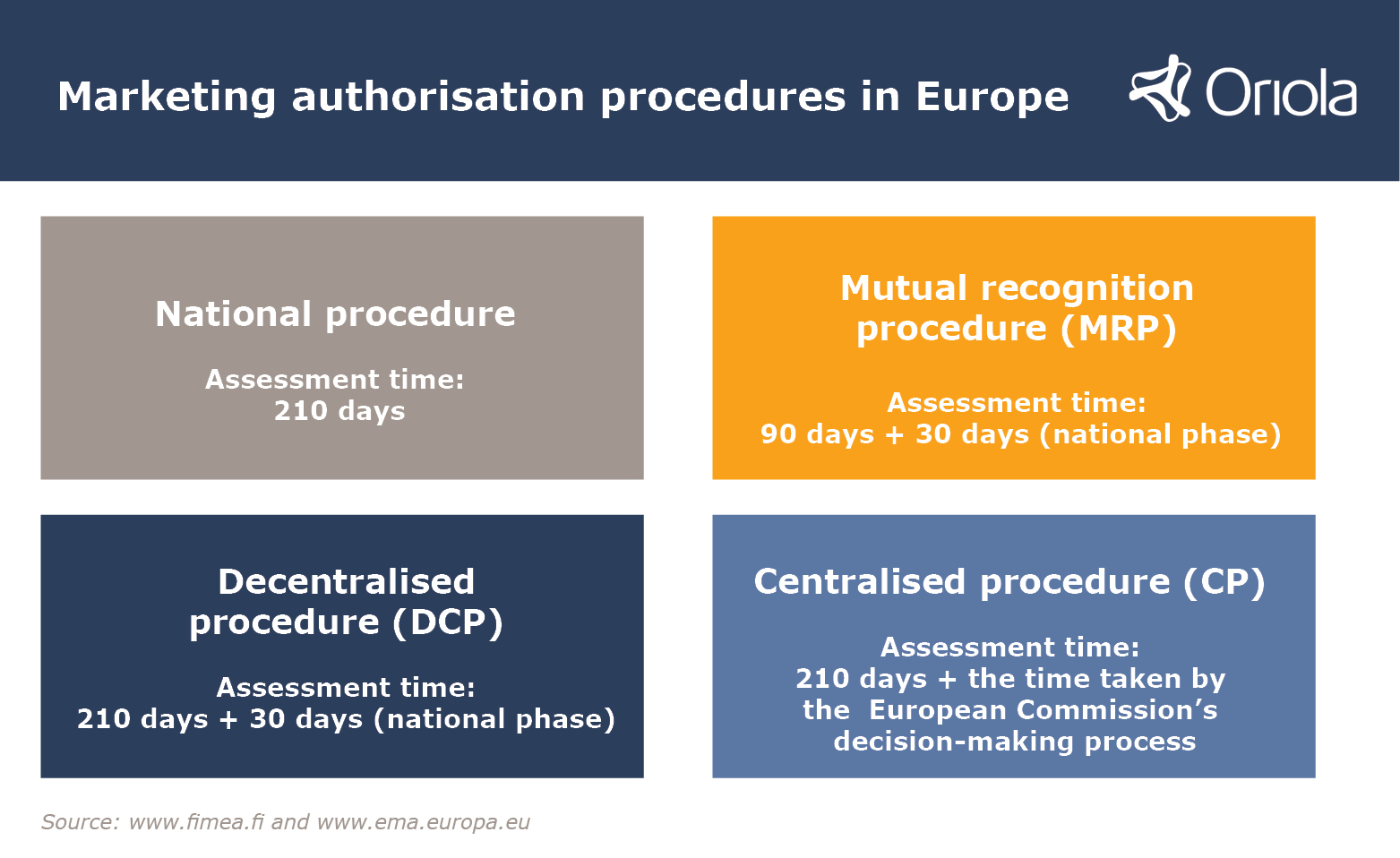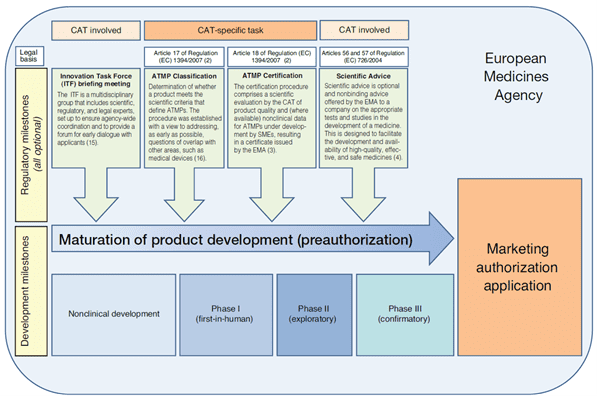
How to design a regulatory strategy to optimize registration of Advanced Therapy Medicinal Products (ATMPs) with European Medicines Agency ? - BlueReg Group

Conditional marketing authorisations give patients access to important new medicines earlier | European Medicines Agency

Eisai submits marketing authorisation application for Lecanemab to the European Medicines Agency | Alzheimer Europe
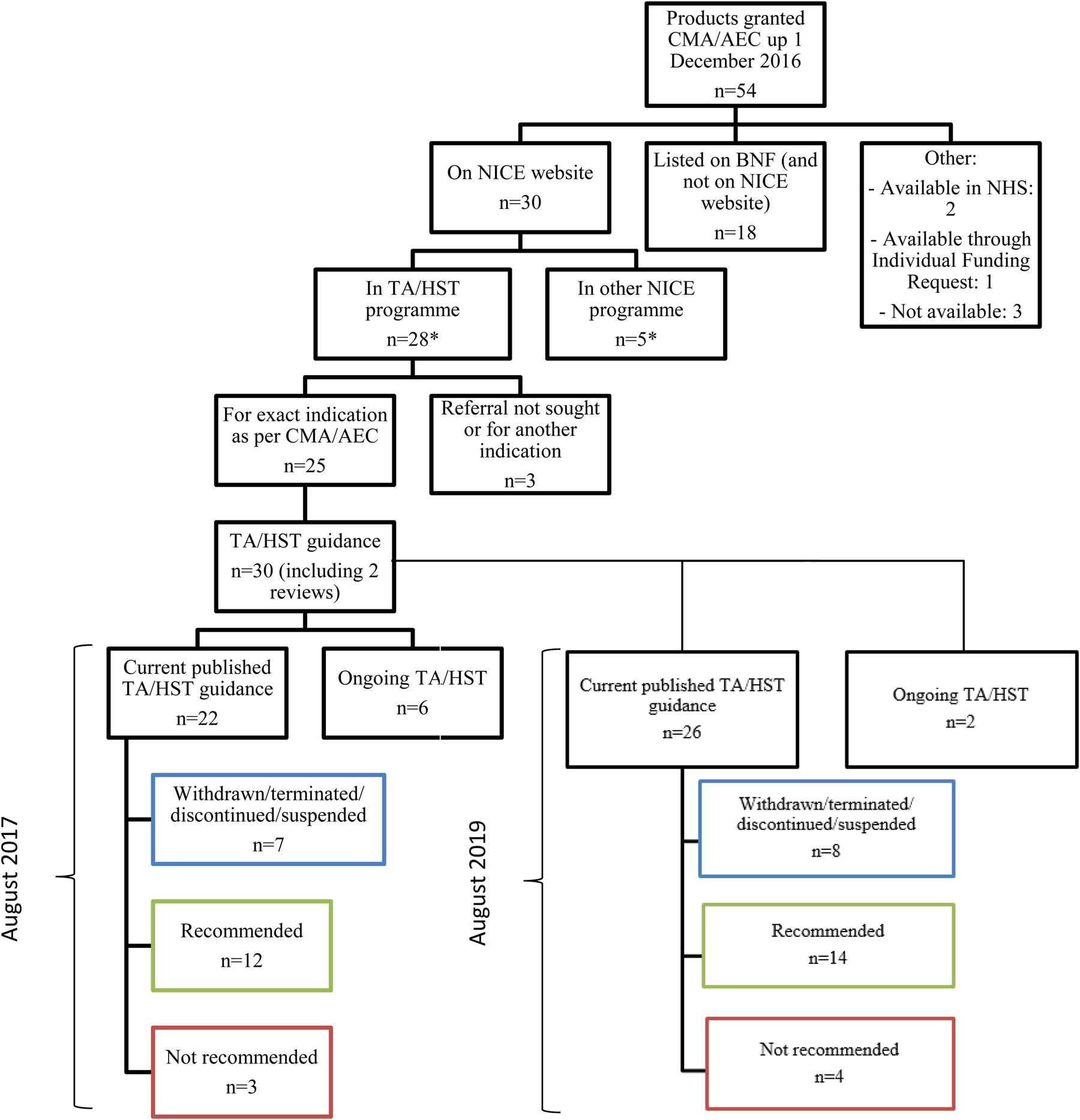
NICE's evaluations of medicines authorized by EMA with conditional marketing authorization or under exceptional circumstances | International Journal of Technology Assessment in Health Care | Cambridge Core

Outcomes of EMA marketing authorization applications: does partnering have an influence? | Nature Reviews Drug Discovery

تويتر \ EU Medicines Agency على تويتر: "EMA receives the application for a conditional marketing authorisation of #COVID19 Vaccine AstraZeneca: https://t.co/FVoelj9uvP https://t.co/rpPoVEcNPk"

Regulatory Pathways to Marketing Authorisation for ATMPs Under the EU... | Download Scientific Diagram

EU Medicines Agency on Twitter: "📢 EMA has just recommended granting a conditional marketing authorisation for the #COVID19vaccine developed by BioNTech/ Pfizer, to prevent #COVID19 in people from 16 years of age.