JCM | Free Full-Text | COVID-19 Vaccines Adverse Reactions Reported to the Pharmacovigilance Unit of Beira Interior in Portugal

6. Perceived Gap Analysis and Research Needs | Current Hazardous Materials Transportation Research and Future Needs |The National Academies Press
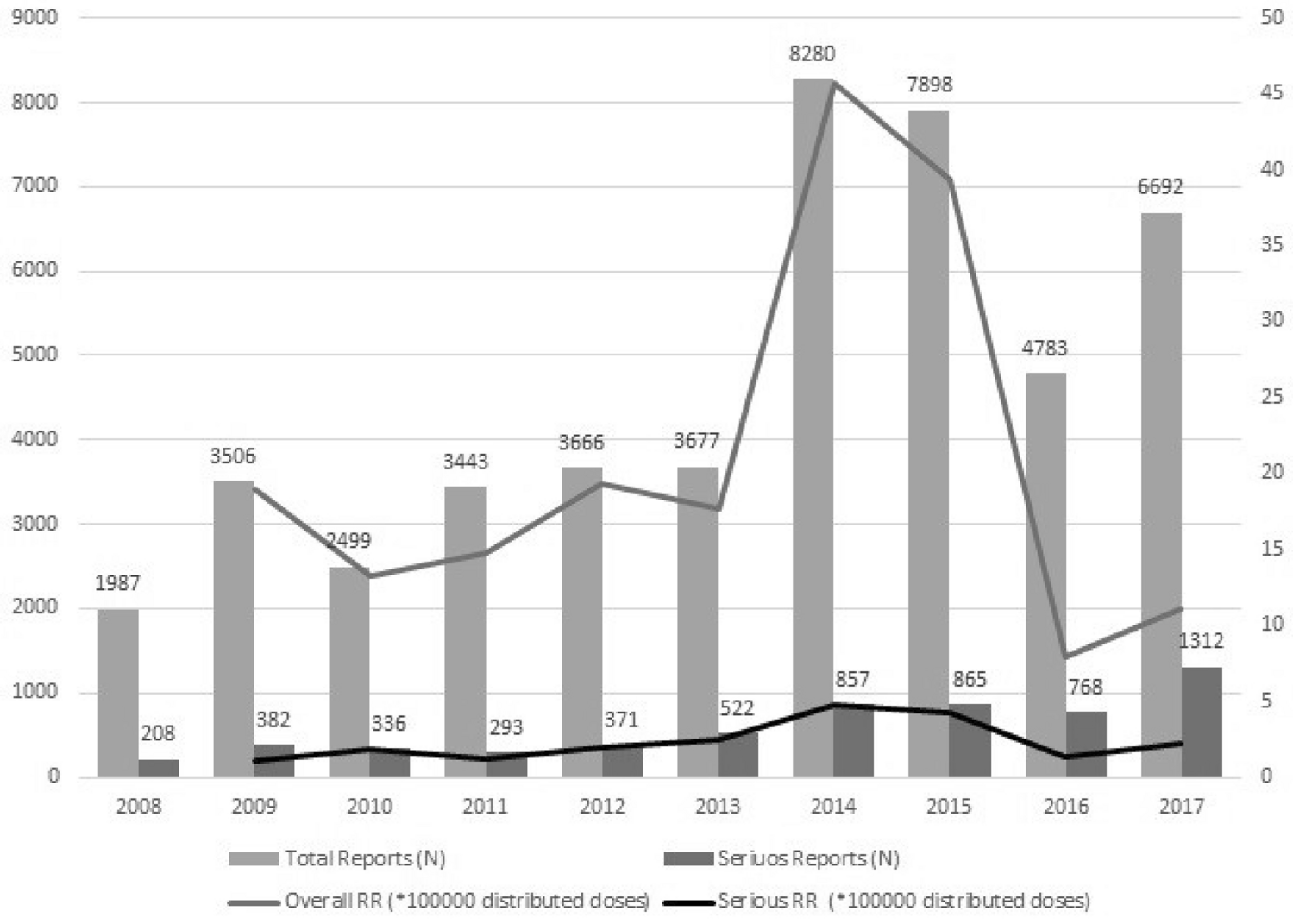
Ten years of vaccinovigilance in Italy: an overview of the pharmacovigilance data from 2008 to 2017 | Scientific Reports

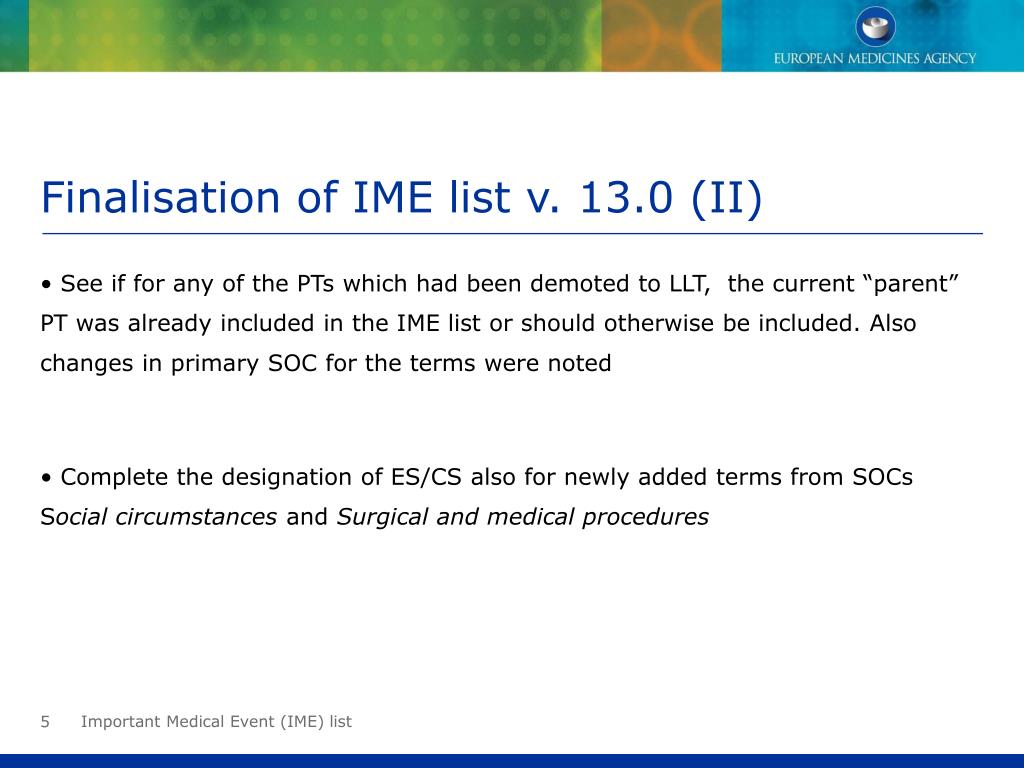
Definitions and difference in PVG terms: Designated medical event (DME), Important medical events (IMEs), WHO critical terms and AESIs
PDF) Health horizons: Future trends and technologies from the European Medicines Agency's horizon scanning collaborations

Access-2-Healthcare SG - EMA Releases EudraVigilance List of Important Medical Event Terms This is a list of important medical event (IME) terms for users of its EudraVigilance safety database, intended to support

Important Medical Events: Current Status and Maintenance Principles | PDF | Medicine | Clinical Medicine


![PDF] Screening for adverse reactions in EudraVigilance | Semantic Scholar PDF] Screening for adverse reactions in EudraVigilance | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/747c892608ff2264a343509c19f362b2bad013f0/34-Table6-1.png)




.jpg)