Impact of the European Union on access to medicines in low- and middle-income countries: A scoping review - The Lancet Regional Health – Europe

PDF) Overview of the European Medicines Agency's Development of Product‐Specific Bioequivalence Guidelines

Medicines for Europe Press release – EMA states no scientific evidence to deter use of ibuprofen in COVID19 patients | Medicines for Europe

Pharmaceutics | Free Full-Text | Drug Safety in Translational Paediatric Research: Practical Points to Consider for Paediatric Safety Profiling and Protocol Development: A Scoping Review

Á1111Ñ Microbiological Examination of Nonsterile Products: Acceptance Criteria For Pharmaceutical Preparations and Substances For Pharmaceutical Use | PDF | Colony Forming Unit | Biopharmaceutical
Concept paper on the revision of annex 1 of the guidelines on good manufacturing practice – manufacture of sterile medicinal p

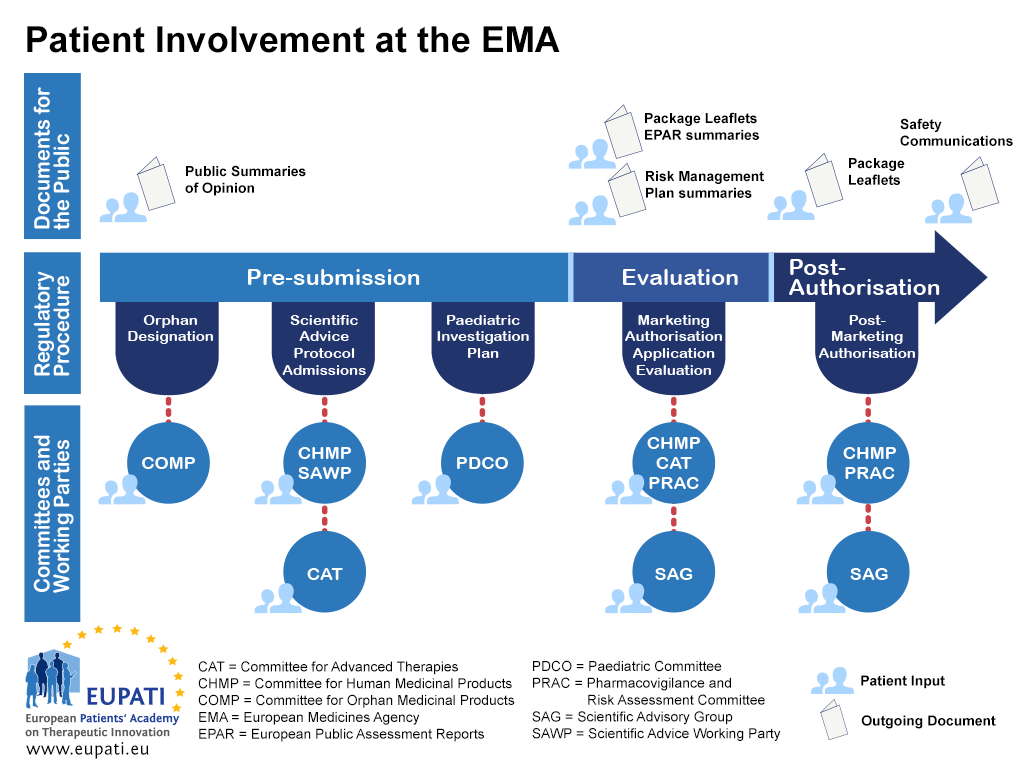
Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations

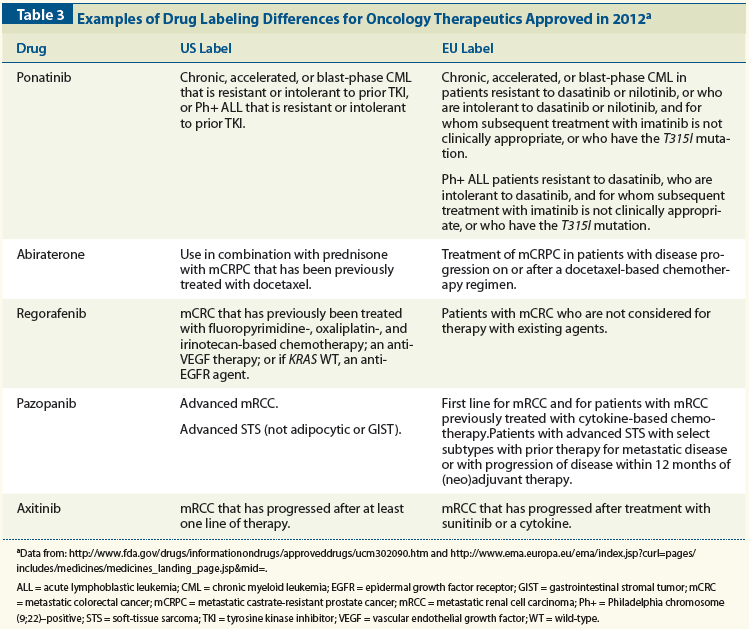
Differences in Evidentiary Requirements Between European Medicines Agency and European Health Technology Assessment of Oncology Drugs—Can Alignment Be Enhanced? - Value in Health

PDF) The EMA Bioanalytical Method Validation Guideline: process, history, discussions and evaluation of its content | Peter van Amsterdam - Academia.edu

Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA
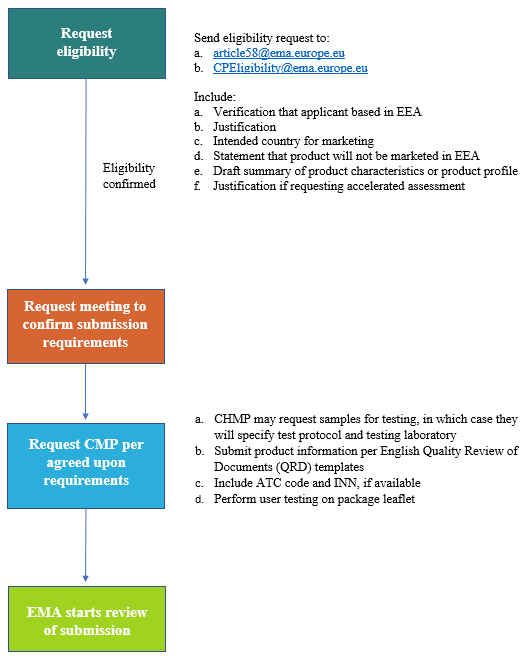
Quality Review of Documents veterinary product-information annotated template (English) version 8 - clean

PDF) Comparison of the EMA and FDA Guidelines on Ulcerative Colitis Drug Development | Klaus Gottlieb - Academia.edu
Global regulatory landscape of biosimilars: emerging and established market perspectives - Document - Gale OneFile: Health and Medicine