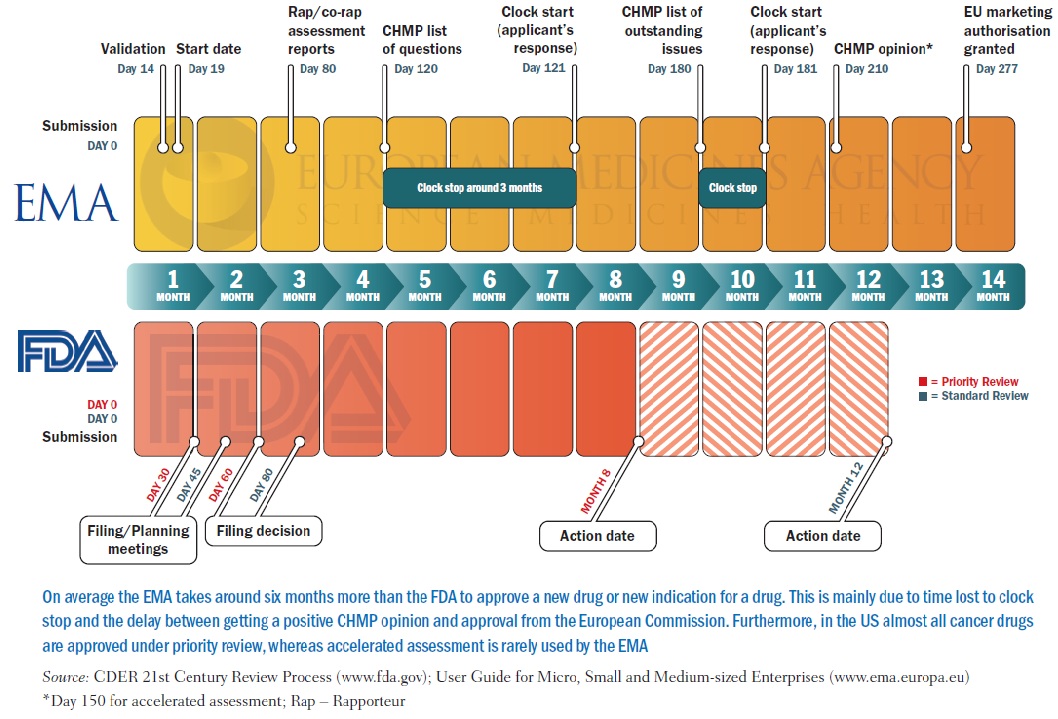
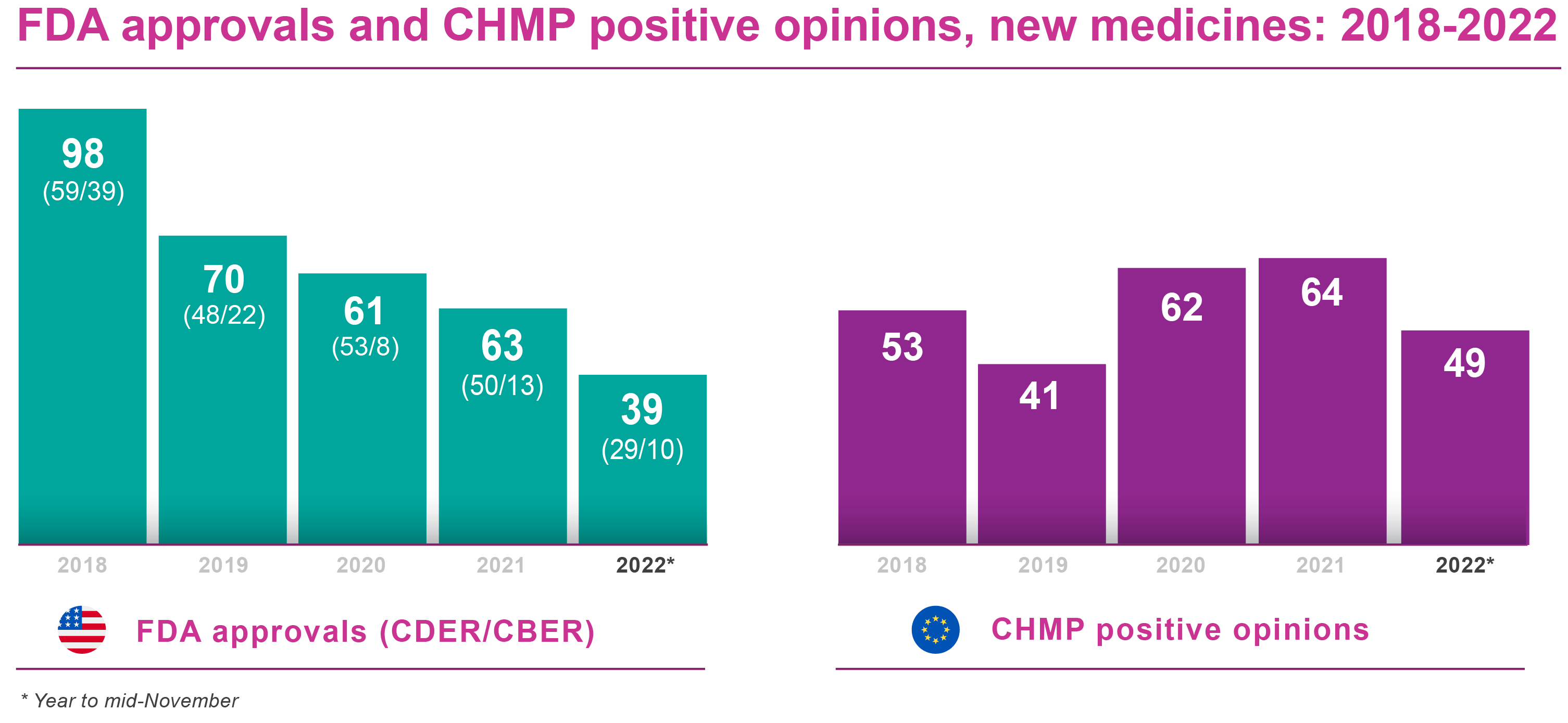
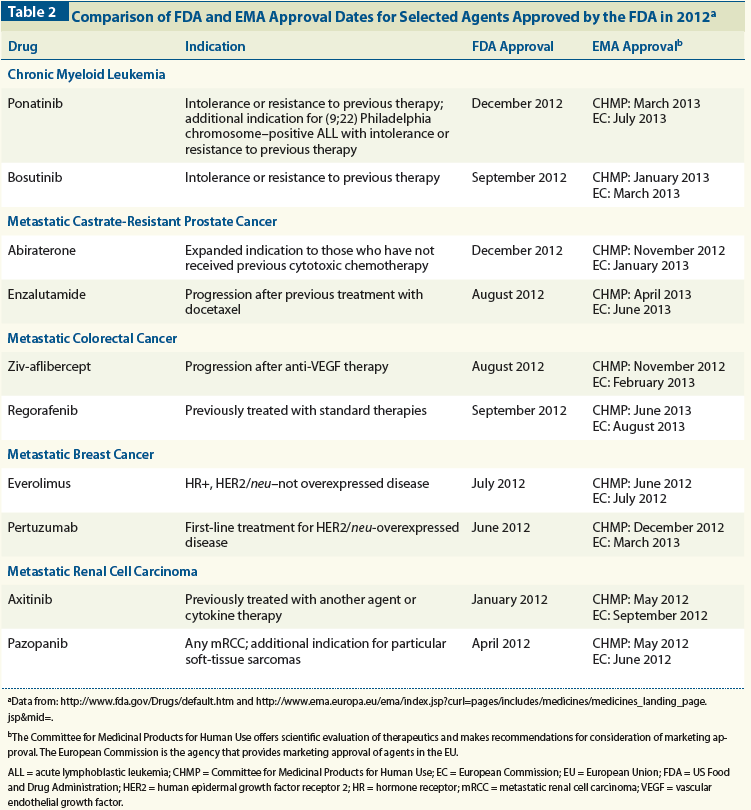
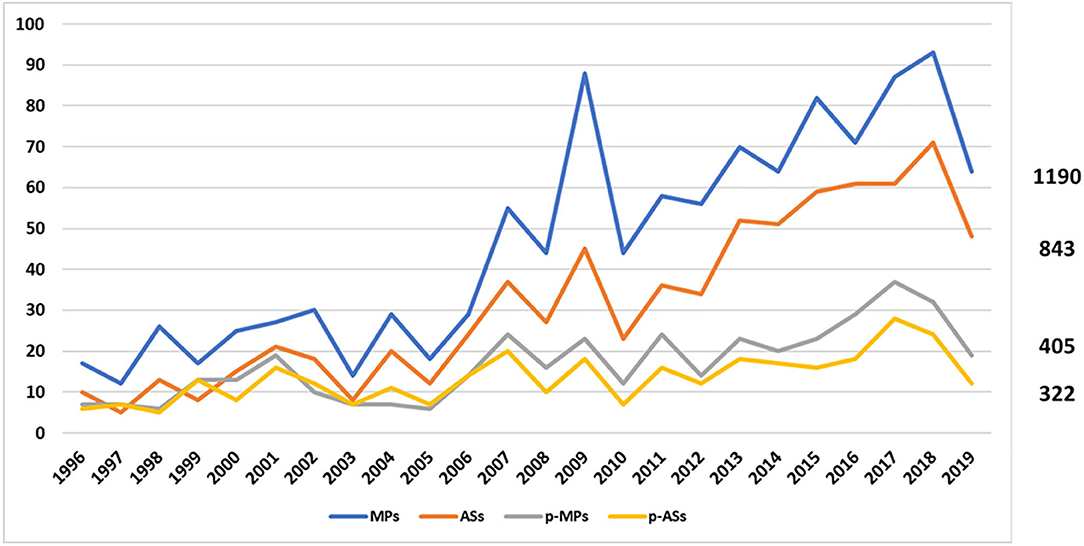
Regulatory approval of pharmaceuticals without a randomised controlled study: analysis of EMA and FDA approvals 1999–2014 | BMJ Open

Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA