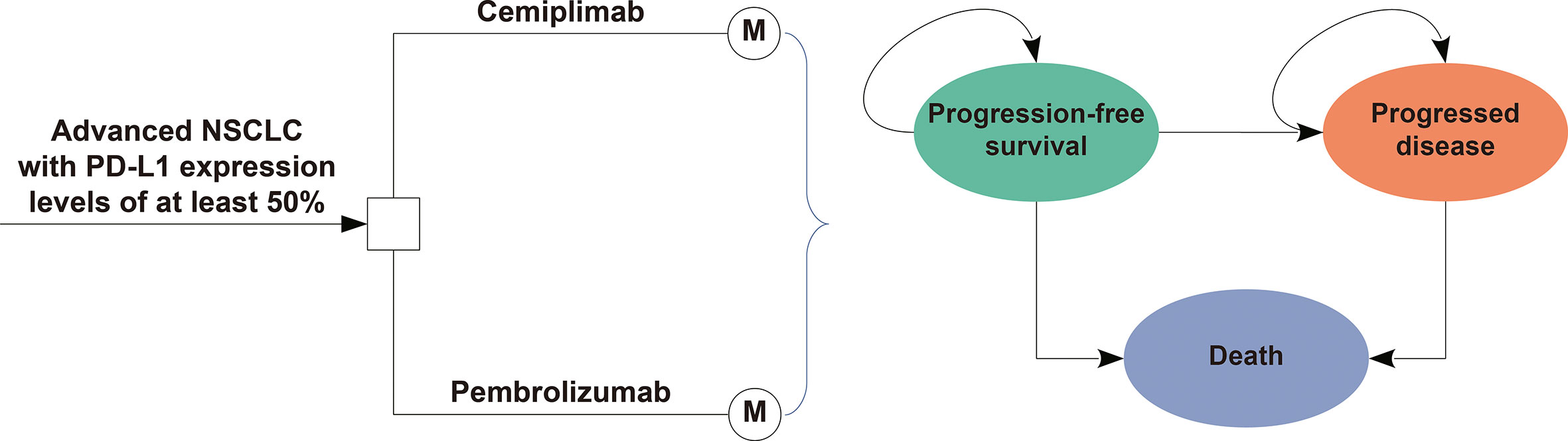
Frontiers | Pembrolizumab vs cemiplimab for the treatment of advanced non-small cell lung cancer with PD-L1 expression levels of at least 50%: A network meta-analysis and cost-effectiveness analysis

Regeneron and Sanofi Present Results of Libtayo (cemiplimab) in P-III EMPOWER-Lung 3 Study as 1L Treatment of Advanced NSCLC at ESMO 2021

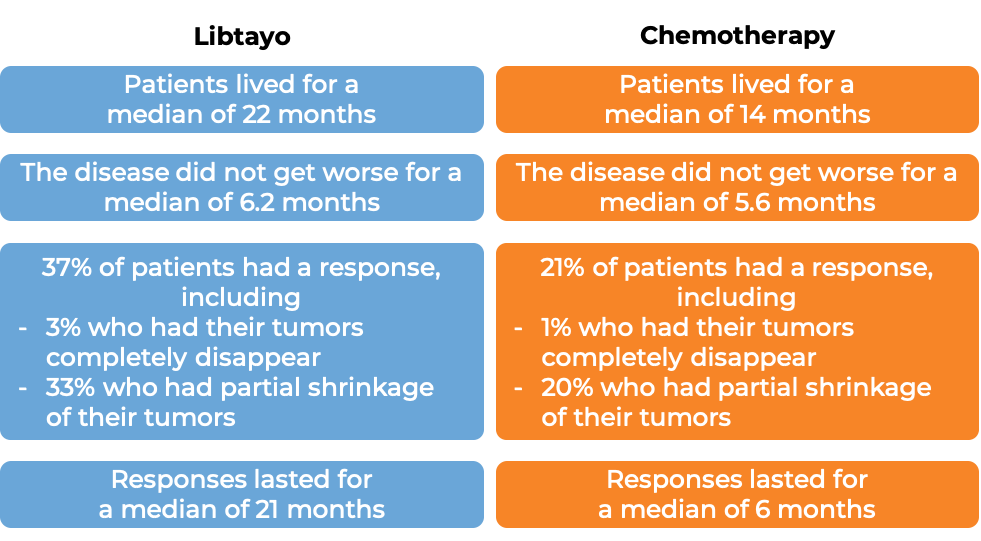
Regeneron's Libtayo (cemiplimab) Receives EMA's CHMP Positive Opinion Recommending Approval to Treat Advanced Cervical Cancer

EMA Committee Recommends Regeneron's Libtayo + Chemotherapy Combo for Lung Cancer - HealthEconomics.Com

Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial - The Lancet Oncology
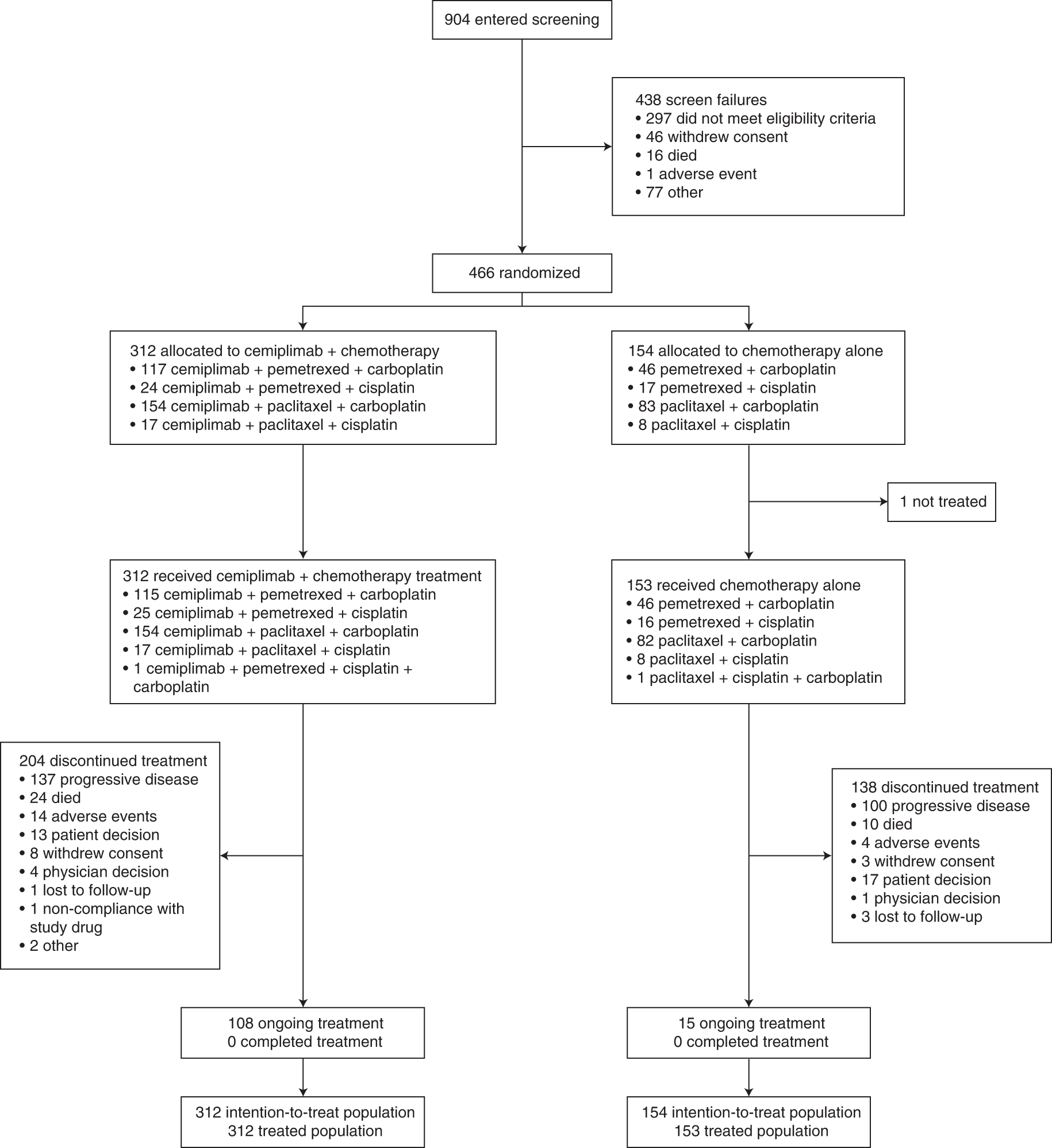
Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial | Nature Medicine
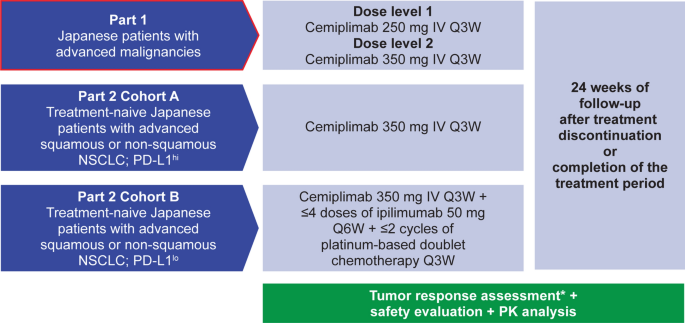
Dose exploration results from Phase 1 study of cemiplimab, a human monoclonal programmed death (PD)-1 antibody, in Japanese patients with advanced malignancies | SpringerLink

Tolerability and antitumor activity of cemiplimab, a human monoclonal anti–PD-1, as monotherapy in patients with pretreated non-small cell lung cancer (NSCLC): Data from the Phase 1 NSCLC expansion cohort - ScienceDirect

Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial - The Lancet Oncology