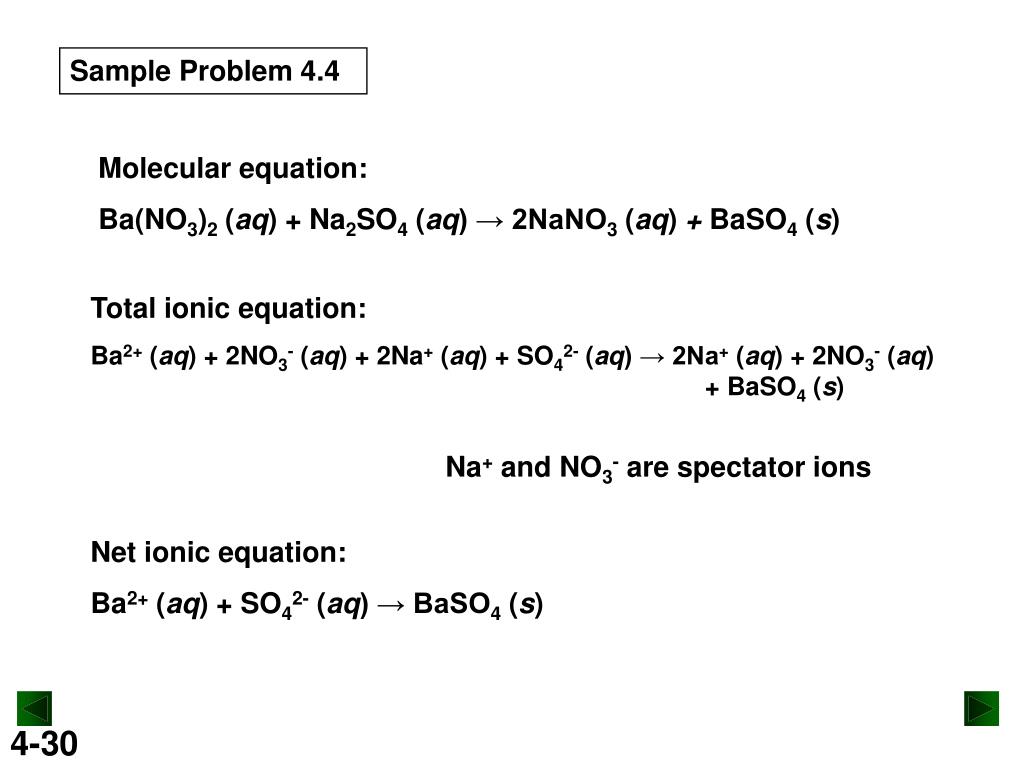
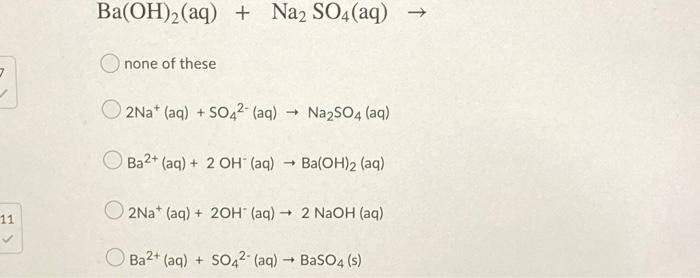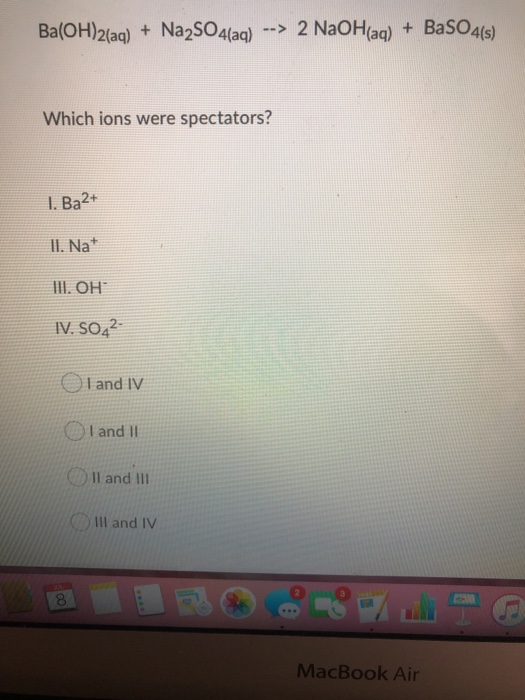SOLVED: What is the net ionic equation if sodium sulfate is mixed with barium hydroxide? 2Ba+(aq) + SO42− (aq) → Ba2SO4(s) Ba2+(aq) + SO42− (aq) → BaSO4(s) Ba2+(aq) + 2OH−(aq) → Ba(OH)2(s)
20 ml of 0.05 M HCL was mixed with 30ml of 0.1M Ba(OH) ↓2. what is the [OH-] concentration in the mixed solution? - Quora

Cрочно : Ba(OH)2+Na2SO4=BaSO4+2NAOH напишите в полном и сокращенном ионном виде - Школьные Знания.com
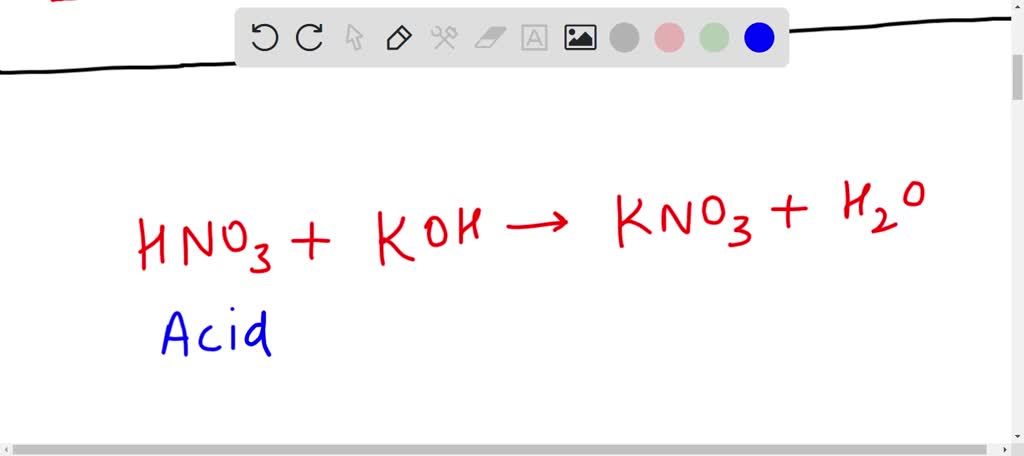
SOLVED: Which of the following equations represents an acid-base neutralization reaction? Group of answer choices A. Ba(OH)2 + Na2SO4 –> BaSO4 + 2NaOH B. HNO3 + KOH –> KNO3 + H2O C.

Na2SO4 + Ba(OH)2 = 2NaOH + BaSO4 Уравнения реакций ионного обмена напишите в полном и кратком - Школьные Знания.com

Nhỏ từ từ đến dư dung dịch Ba(OH)2 lần lượt vào các dung dịch sau: NaHCO3, AlCl3, NaHSO4, (NH4)2CO3, FeCl3, Na2SO4 và KN?
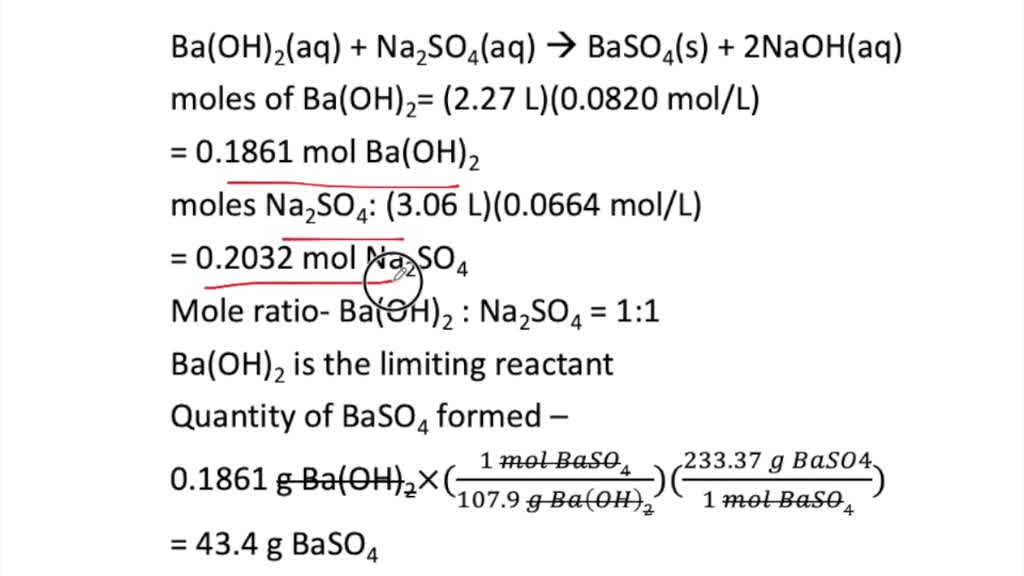
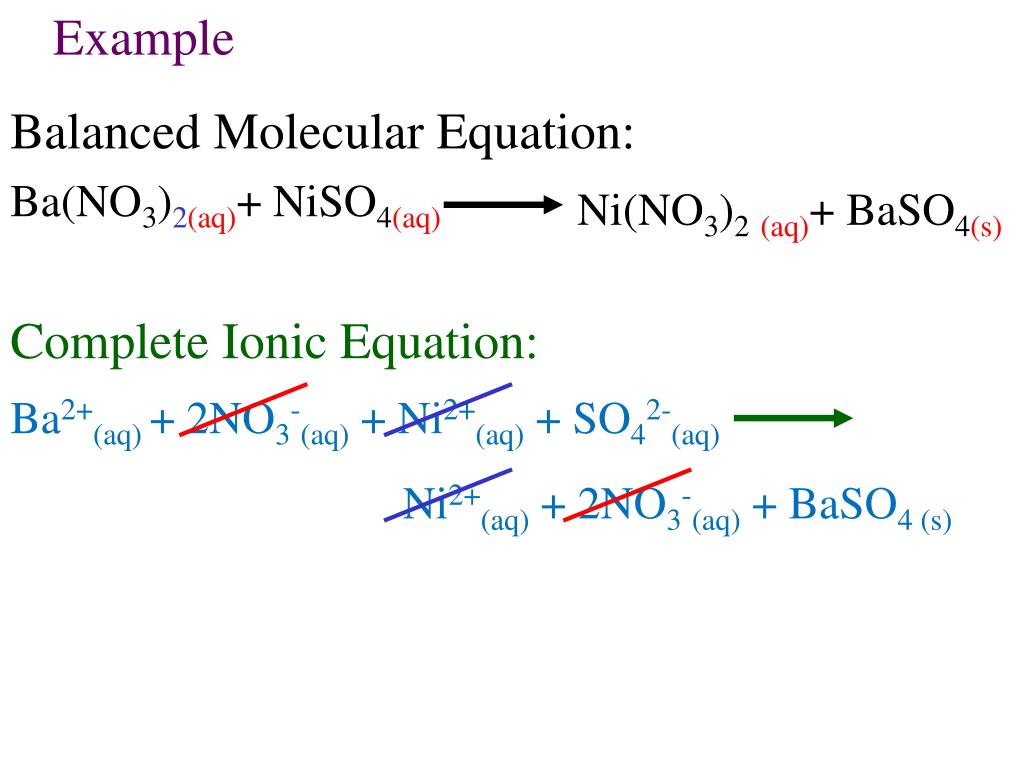
SOLVED: Calculate the mass of the precipitate formed when 2.27 L of 0.0820 M Ba(OH)2 are mixed with 3.06 L of 0.0664 M Na2SO4.